Aim: To perform staining of tissue sections using antibodies (immuno histochemistry)
Principle: Antigen - antibody interactions form the basis of several techniques used in modern day scientific research and in routine clinical diagnosis. One such technique immunohistochemistry is used for the localization of an antigen in a cell or tissue using a specific antibody. The antibody binds specifically to the antigen present in the cell and the antigen-antibody complex is detected using an enzyme - linked secondary antibody. Addition of the substrate for the enzyme forms an insoluble colored precipitate in the tissues and allows localization of antigens.In Immunohistochemistry, sections of fixed, paraffin embedded tissue of interest are taken on glass slides. Fixation preserves the morphology of the cells/tissues as well as keeps the antigen from degrading. Paraffin has to be removed in order to allow the antibody to penetrate the tissue cells and bind to the antigen. This is achieved by using xylene. Antibodies are liquids and will not bind to the antigen unless the dry sections are hydrated. Hence the need to gradually introduce water in the cells of the tissues through grades of alcohol. Phosphate Buffered Saline (PBS) maintains the physiological pH ideal for any antigen-antibody reaction. The blocking step avoids any non-specific binding of the primary antibody. Most tissues express the enzyme Horse Radish Peroxidase endogenously. Hence H2O2. PBST and PBS washes remove excess of unbound antibody (Primary/Secondary) and maintain the PH.
Procedure:
Day 1: Deparafinizing, rehydrating and blocking
Deparaffinize the sections by placing the slide in Coplin jar with xylene for 10 minutes.
Transfer to a second Coplin jar of Xylene and keep the slides for 10 minutes.
Rehydrate the sections by passing the slide through various grades of alcohol viz. Absolute, 90%, 80% and 70% for 10 minutes in each jar.
Dip the sections in 1X PBS.
Place the slide in a moist chamber with sections facing upwards and cover each section with 100 µl of blocking serum. Incubate for 1 hour at room temperature (RT).
Rinse the slide in a jar of PBS.
Return slide to the moist chamber. Block endogenous peroxidase in the tissue sections by covering each of the sections with 100 l of freshly prepared mixture of methanol and 3% H2O2 in the ratio 4:1. (Add 200µl methanol to 50 µl 3% H2O2 in a 1.5 ml vial). Incubate for 30 minutes at RT.
Rinse the slide in a jar of fresh PBS.
Return slide to the moist chamber. To one section add 100µl of primary antibody. To the other section add 100 µl of negative control antibody. Incubate overnight at 4°C. (Cover the box and leave it in the refrigerator). Please note the orientation of the Sections in your observation book.
Day 2: Staining and mounting
Drain the antibody solutions on tissue paper. Place in a coplin iar with PBST. Wash 3 times with fresh PBST for 10 minutes each, followed by 3 washes in a fresh jar of PBS for 10 minutes each. (You may keep the coplin jar on a rocking platform).
Place the slide in the moist chamber. Add 100 pi of djjuted secondary antibody to each section and incubate for 1 hour at RT. (Do not exceed 1 hour incubation. It can increase non-specific reaction).
Transfer the slide to fresh PBST and wash for 10 minutes. Give 3 washes of 10 minutes each, followed by 3 washes with PBS of 10 minute each.
Wipe excess PBS around the sections. Keep the slide on a flat surface. Add 100 µl of substrate to each section and observe for colour development. (Do not exceed 10 minutes. Avoid exposure to direct light).
Stop the reaction by placing the slide in a jar of PBS.
Counter stains the sections by adding 100 µl of Hematoxylin to each section for 30 seconds.
Wash the slide under slow running tap water for 5 minutes by placing them in a coplin jar.
Dehydrate the sections by passing them through increasing grades of alcohol viz., 70% alcohol, 80% alcohol, 90% alcohol and absolute alcohol for 10 minutes in each.
Clear the sections by placing them in a jar of Xylene for 10 minutes.
Transfer to a second jar of Xylene for 10 minutes.
Mount the sections by placing a drop of DPX mountant on them and slowly dropping a coverslip on it (no air bubbles should be present).
Allow drying for half an hour.
Observe the staining under a light microscope.
Observation:
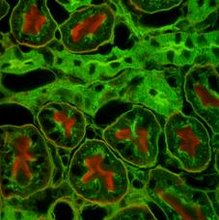
Observe the staining pattern of epithelial cells in both the sections and note the differences. The margins of epithelial cells show brown staining with primary antibody. Negative control antibody does not show this pattern. Nuclei of all cells stain blue with Hematoxylin.
Interpretation:
In the section stained with the primary antibody, the margins of most of the epithelial cells are stained brown indicating the presence of the antigen in the membranes of epithelial cells. This staining pattern is not observed in the section stained with the negative control antibody. The primary antibody specifically localizes the antigen present in the membranes of epithelial cells.
Subscribe to:
Post Comments (Atom)

3 comments:
Valuable information and excellent design you got here! I would like to thank you for sharing your thoughts into the stuff you post!! Thumbs up!
Micropipette
hai good mor reallly its so useful but calculation part is missing try to do that too
I had visited your website which was really good Flask
Post a Comment